Seemingly trivial things that intrigue you
Comments
-
Just replacing the cable for the front chainring (well, it was nine years old, as per supplied on the new bike), and I had to keep on pulling 'fluff' off it to stop it wedging as I pulled it back through, as if it had been coated with something and the coating had broken up. Never had that before. Weird.
Anyway, off for a ride over two 1300m+ cols after lunch. Sun cream advised.
0 -
It is teflon. It is an irritating thing they do to reduce friction on some cables. I try to avoid those cables because it actually makes things worse after a while.
0 -
Aha, thanks. To be fair, it was good going that the cable had lasted that long, but it caught me out as when I pulled the old one out through the internal sheath in the frame, it got stuck in the sheath good and proper, and I had to remove it from the frame and make a subtle cut in the top of the sheath to get it out. It would explain why the front shifter had got quite sticky - I'd thought it was the derailleur itself playing up, but obviously it wasn't: now with the new, normal cable, it's as good as new.
0 -
BTW, my two 1300m+ cols turned into just one 1040m one, as the weather in the planned direction didn't look as promising. 🐔
0 -
What a great VTOL design this is.
0 -
Amazing!
2 -
Flicking through the TV channels this morning while having breakfast and ended up watching Border Patrol. I really couldn’t understand the lengths people go through to smuggle food (mainly from south-east Asia) into New Zealand. We’re not talking about commercial quantities to be sold just a few mangoes and strips of meat hidden with dried squid (also who would eat meat that has been transported like that?). I’ve seen it before where people get stopped with all sorts of food in their luggage - I thought Brits were bad for taking their home foods away but this is a whole different level.
1 -
I use Facebook market place now and then, and because i do, I get updates on certain items I've searched for in the past. One was telescope eyepieces.
I've mentioned algorithms before so this is partly intriguing, but one advert that does pop up among the various telescope bits is a one for 'hair and makeup'.
The intriguing bit about this advert is that features a young lady who look a bit like Gemma Collins (yuck!). She is a sort of browny-orange, has lips like two hot-dog sausages and eyebrows that appear to be two lengths of insulating tape. If this is what she does to herself(?) then how the hell does she get any business? If it's one of her clients and this is what she considers to be her best work for an advert, how the hell does she get any business?
Intrigued.
The older I get, the better I was.0 -
The food in NZ isn't even that bad 😄
0 -
Being a slob (and being more interested in polishing off the Côtes de Rhône and social media stuff) I'd not washed up the pan I used to steam carrots last night. I was just about to sling the water down the sink, but was rather taken aback by its colour. And yes, they were orange carrots. Any explanation?
0 -
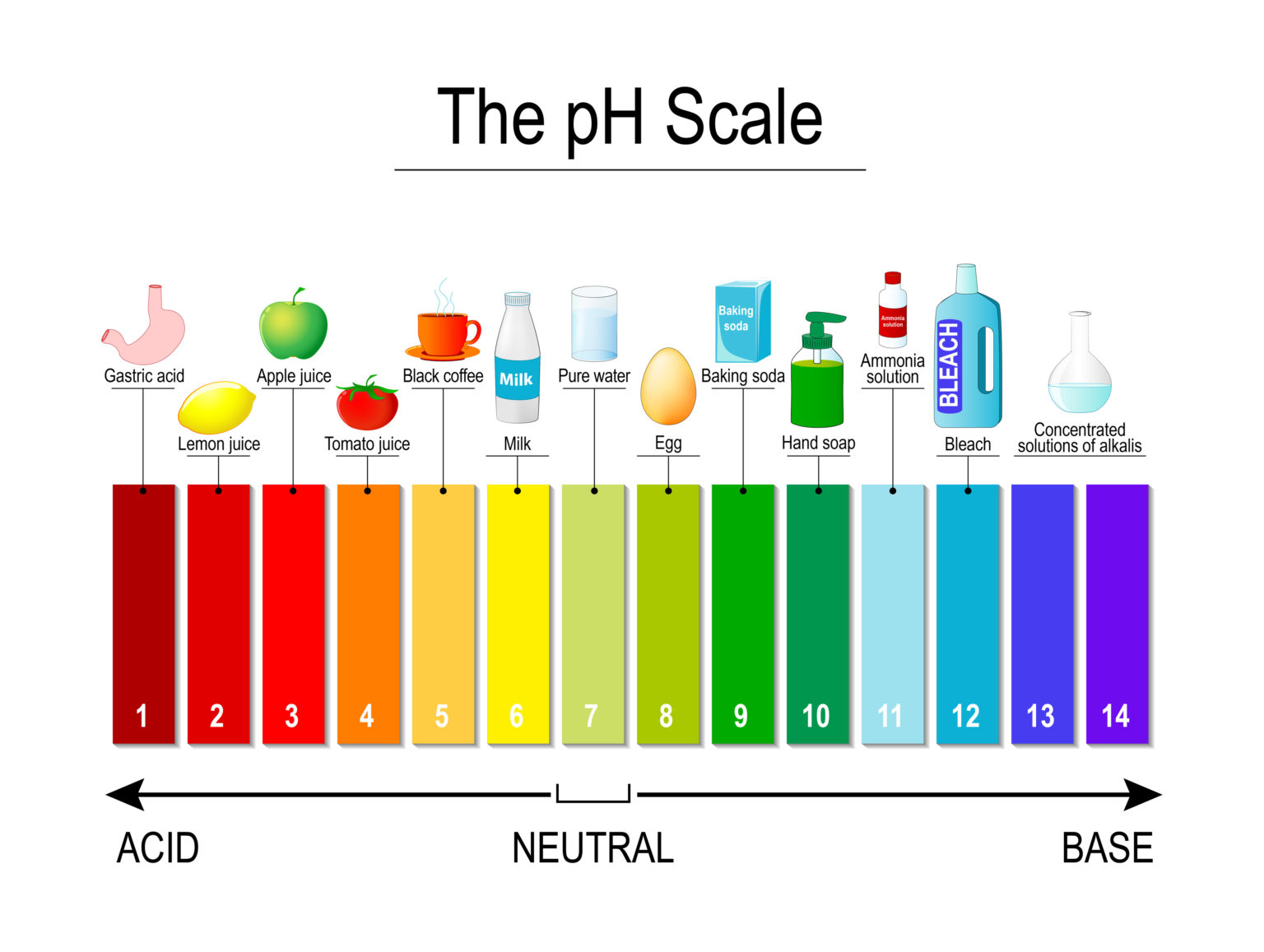
I did a homework science project with my daughter where we boiled red cabbage and used the water as an indicator, it changes colour when you add an acid.
Maybe carrot juice does something similar?
0 -
I did some searching.
Two possibilities - (1) you didn't take the green parts at the top off, and you have basically distilled the chlorophyll in the mould at the top or the leaves.
Or (2) somehow the carotenoids have oxidised. I'd have thought it more likely that would remove colour entirely, but it is one of those things where you can find the answer you think you want by appropriate searching, and so I did pick up some articles discussing isomerisation leading to colour changes. Even those seem to be more to the red end of the spectrum though.
So I am guessing option (1) - here's a picture randomly selected of chlorophyll solutions. Look familiar?
0 -
I did cut the tops off, but maybe not enough, though all the cooked carrots are completely orange. Yes, those greens do look the same (I'd guess at about 1% for the same shade as mine).
0 -
I thought it might be a reaction (oxidation) with an aluminium pan. A Google revealed, 'Why has my carrot cake turned green?' Their thoughts, aluminium pans or by adding sodium bicarbonate (alkaline).
0 -
-
Going back to my KS2 chemistry, the cabbage solution shows green for a pH of around 9 or 10 so if you managed to make a carrot version, then splashed hand soap in, you would get the green colour.
Apparently the cabbage indicator is caused by a pigment called anthocyanin, google suggests that purple carrots contain a lot but no mention of it in orange carrots.
If you still have the mystery liquid, chuck a load of lemon juice into it and see what happens.
https://www.science-sparks.com/make-a-red-cabbage-indicator/
0 -
No lemon juice in the house, but am tempted to get some citric acid to chuck in... I've put the liquid in the fridge for now, as I'm intrigued.
0 -
I'm intrigued why you have citric acid in the house but not lemon juice?
0 -
Hard water (mineral rich, high alkalinity) from your supply?
0 -
Citric acid/lemon juice stops browning of fruit (apples) by slowing oxidation and preserving colour.
0 -
I have neither. Just thought that citric acid would be a 'purer' experiment, and can always be used to give extra piquancy to things.
0 -
Very. Straight off the limestone plateau. Just UV treated.
0 -
That tallies with the green carrot cake theory of it being sodium bicarbonate. I might grate some carrot later and add some sodium bicarbonate (baking powder) to the mix. I will leave some unadulterated as a control, otherwise @First.Aspect (being a chemist) might question any results.
1 -
Limestone is mostly calcium carbonate, which is poorly soluble in water. That's not it.
Sodium bicarbonate is a buffer. It neutralises acids, but that's not quite the same as saying it makes a solution alkaline. It also decomposes when heated, which in Brian's experiment would have resulted in a carbonate, which IS alkaline.
So it is feasible - if one assumes that the reaction of carotene and hydroxide makes it go green.
That's more of a struggle for me to understand, i.e. I don't. I would have thought it would go the other way, because you are promoting hydrolysis and breaking down what is called a conjugated system. I suppose it could be more subtle than that and why I stumbled across references to isomerisation, which essentially means rearranging the system.
Too hand wavy. Would be interested in whether you get green carrots by adding bicarb.
0 -
I look forward to seeing your homework report Brian 😃
0 -
Well, in my laziness, I tried the lemon 'sirop' I had in house anyway...
1 -
0
-
-
0