Extending the life of lithium ion batteries
RutlandGav
Posts: 144
I sometimes throw opinions and ideas about cycling on this board that are a bit "out there" and get ripped to pieces by the old hands.
When it comes to electronics however, i'm on safer ground so i thought i should share this :
What causes lithium ion batteries to lose capacity?
Traditionally, manufacturers quote cell lifespan in terms of charge/discharge cycles. However, modern lithium ion chemistries suffer such little capacity loss from each cycle that we can practically ignore it.
What really matters is "Calendar Fade". In layman's terms, over time the battery "rots" internally due to unwanted chemical reactions. The rate of these reactions is chiefly driven by two factors
a) State of charge. The higher the state of charge, the quicker the degradation process. Below 65%, capacity loss is so slow the cell is likely to outlive the device it is plugged in to. Above 80%, the reaction rates increase exponentially, so that a battery stored at 100% can loose over 30% per year.
b) Temperature. The lower the better.
Lead acid car batteries behave differently - the lower the state of charge, the faster they degrade, so it is important to keep them topped off. Unfortunately this leads to people treating their Li-ions in the same way.
Some laptops and some electric vehicles allow the user to set what level to charge your battery to. Unfortunately this feature, which costs almost nothing to implement, has yet to trickle down to manufacturers of bicycle lights. Many of these devices even lack any kind of state of charge indication, so you can manually intervene in the process.
What you can do
1) Charge just before use
If you only use the lights once a week, the natural inclination might be to charge them up at the end of your ride then put them away in a cupboard till next week, fully charged. This is the worst thing you can do of course. If you charge just before the ride, your battery spends less time at the harmful 100% level. If they take a long time to charge, you could compromise by keeping them part charged and do the final top off just before the night ride. It's only the time spent above 75% that does significant harm to the battery.
2) Cool it
The best place to keep them when not in use is probably your fridge. When inactive, the cells don't really care about being frozen either, however it is very important they stay above freezing temperatures when being charged. At low temperatures, the electrolyte freezes and the lithium ions can't move about. On discharge, this is harmless to the battery but may render them unable to deliver current quickly enough to keep your device running - inconvenient. When charging however, frozen electrolyte does the same kind of damage that overcharging the battery does - permanent capacity loss, and lithium metal deposits forming on the surface of the anode. Lithium metal is not supposed to be present in a lithium ion battery - all the lithium (which is only 5% of the cell by weight, btw) should be dissolved in either the cobalt oxide cathode (when discharged) , the electrolyte , or the graphite anode (when charged). The presence of lithium metal deposits renders the battery prone to spontaneous catching fire and other fun stuff.
3) The (charger) withdrawal method
If you are lucky enough to have a device that indicates its state of charge, and are headed out on a shorter ride, you could unplug the lights before they charge to 100% full. This is aided by the fact that the charging process slows down as the battery gets fuller. Below 75%, the charge rate is limited by the power of your charger. Cramming the last 25% in however, can take 45 minutes or more no matter how good the charger may be, since you are limited by the battery.
Here's an example with scary amounts of electricity flowing -
https://www.youtube.com/watch?v=S00RG6uqpPo
Caveats - Long Term Storage
Lithium ion cells themselves have no self discharge. A bare cell put in a cupboard with 1% charge will still have 1% 3 years later. However the device they are attached to will often drain a small amount of power even when "off". Even if you remove the battery from the device, many single cell batteries, and ALL multi-cell packs , contain a battery protection circuit which exerts a slight drain.
If the battery is overdischarged below 0%, then at approximately 3V the charger may refuse to attempt to recharge the thing. 'Elf and Safety and all that. They'd rather you just buy a new set of lights or battery off them. If there is a battery protection circuit in the way, it will shut down at some point and fail safe to the "battery disconnected" state - meaning you can neither charge nor discharge it.
So, if you're planning to leave it unused for a long time, don't put it away Completely flat. For this reason lithium ion batteries are normally shipped at 30%. The actual state of charge it is OK to store at depends on how often you're going to check up on the thing, and make sure it's ok.
When it comes to electronics however, i'm on safer ground so i thought i should share this :
What causes lithium ion batteries to lose capacity?
Traditionally, manufacturers quote cell lifespan in terms of charge/discharge cycles. However, modern lithium ion chemistries suffer such little capacity loss from each cycle that we can practically ignore it.
What really matters is "Calendar Fade". In layman's terms, over time the battery "rots" internally due to unwanted chemical reactions. The rate of these reactions is chiefly driven by two factors
a) State of charge. The higher the state of charge, the quicker the degradation process. Below 65%, capacity loss is so slow the cell is likely to outlive the device it is plugged in to. Above 80%, the reaction rates increase exponentially, so that a battery stored at 100% can loose over 30% per year.
b) Temperature. The lower the better.
Lead acid car batteries behave differently - the lower the state of charge, the faster they degrade, so it is important to keep them topped off. Unfortunately this leads to people treating their Li-ions in the same way.
Some laptops and some electric vehicles allow the user to set what level to charge your battery to. Unfortunately this feature, which costs almost nothing to implement, has yet to trickle down to manufacturers of bicycle lights. Many of these devices even lack any kind of state of charge indication, so you can manually intervene in the process.
What you can do
1) Charge just before use
If you only use the lights once a week, the natural inclination might be to charge them up at the end of your ride then put them away in a cupboard till next week, fully charged. This is the worst thing you can do of course. If you charge just before the ride, your battery spends less time at the harmful 100% level. If they take a long time to charge, you could compromise by keeping them part charged and do the final top off just before the night ride. It's only the time spent above 75% that does significant harm to the battery.
2) Cool it
The best place to keep them when not in use is probably your fridge. When inactive, the cells don't really care about being frozen either, however it is very important they stay above freezing temperatures when being charged. At low temperatures, the electrolyte freezes and the lithium ions can't move about. On discharge, this is harmless to the battery but may render them unable to deliver current quickly enough to keep your device running - inconvenient. When charging however, frozen electrolyte does the same kind of damage that overcharging the battery does - permanent capacity loss, and lithium metal deposits forming on the surface of the anode. Lithium metal is not supposed to be present in a lithium ion battery - all the lithium (which is only 5% of the cell by weight, btw) should be dissolved in either the cobalt oxide cathode (when discharged) , the electrolyte , or the graphite anode (when charged). The presence of lithium metal deposits renders the battery prone to spontaneous catching fire and other fun stuff.
3) The (charger) withdrawal method
If you are lucky enough to have a device that indicates its state of charge, and are headed out on a shorter ride, you could unplug the lights before they charge to 100% full. This is aided by the fact that the charging process slows down as the battery gets fuller. Below 75%, the charge rate is limited by the power of your charger. Cramming the last 25% in however, can take 45 minutes or more no matter how good the charger may be, since you are limited by the battery.
Here's an example with scary amounts of electricity flowing -
https://www.youtube.com/watch?v=S00RG6uqpPo
Caveats - Long Term Storage
Lithium ion cells themselves have no self discharge. A bare cell put in a cupboard with 1% charge will still have 1% 3 years later. However the device they are attached to will often drain a small amount of power even when "off". Even if you remove the battery from the device, many single cell batteries, and ALL multi-cell packs , contain a battery protection circuit which exerts a slight drain.
If the battery is overdischarged below 0%, then at approximately 3V the charger may refuse to attempt to recharge the thing. 'Elf and Safety and all that. They'd rather you just buy a new set of lights or battery off them. If there is a battery protection circuit in the way, it will shut down at some point and fail safe to the "battery disconnected" state - meaning you can neither charge nor discharge it.
So, if you're planning to leave it unused for a long time, don't put it away Completely flat. For this reason lithium ion batteries are normally shipped at 30%. The actual state of charge it is OK to store at depends on how often you're going to check up on the thing, and make sure it's ok.
0
Comments
-
Thanks, that is very helpful and interesting!If the battery is overdischarged below 0%, then at approximately 3V the charger may refuse to attempt to recharge the thing. 'Elf and Safety and all that. They'd rather you just buy a new set of lights or battery off them. If there is a battery protection circuit in the way, it will shut down at some point and fail safe to the "battery disconnected" state - meaning you can neither charge nor discharge it.
I have this with my phone, and I guess this may explain it. It didn't seem to be a problem when the phone was newer - it's now 2 years old - but it now seems to be that if I run it till it shuts down for lack of battery, when I put it on charge the screen shows 0% and indicates that it's charging but it never goes above 0%. The solution seems to be charging it on a transformer with a slightly higher power output than the transformer it was supplied with, which seems to kickstart it.0 -
Many thanks0
-
Sourcing quality cells makes a difference too. There are so many fakes on the market, which are either very old recycled cells with new tops and shrink wraps or simply sleeves on cheaper cells. Avoid cells claiming 5,000mAh capacity, very few cells can deliver more than 2,800mAh at any reasonable discharge (e.g. a typical 2A LED based light)
Always check the weight against the manufacturer specification - fakes are often lighter
Fakes often have a hollow clay pot sound if you tap them against each other.
They should not get hot when charging - warm is OK.
They should not charge faster than their specification - i.e. if you have a 500mA charger, a 2.5Ah cell is going to take around 5 hours to charge from empty.
You will often find really good cells in old laptops.
for example
5Ah claimed - 1Ah in reality even at low current.
and then there are the really dangerous fakes: 0
0 -
Sourcing quality cells makes a difference too. There are so many fakes on the market, which are either very old recycled cells with new tops and shrink wraps or simply sleeves on cheaper cells. Avoid cells claiming 5,000mAh capacity, very few cells can deliver more than 2,800mAh at any reasonable discharge (e.g. a typical 2A LED based light)
https://www.youtube.com/watch?v=pxP0Cu00sZs
If you're up for a VERY in depth explanation watch the above lecture. Yep, a third factor in battery lifespan is out of your hands - whether or not the manufacturer included additives in the electrolyte , which prevent it from breaking down over time, when stored at high voltages and temperatures. When the electrolyte breaks down, it leaves solid deposits on the surface of the anode and cathode material, which have a sponge-like granular structure. Eventually, these deposits plug the holes in the sponge-like structure such that the lithium ions can't diffuse in and out fast enough to run high drain devices. Aged batteries will often run the light on super economy setting almost as long as when new, but on a high brightness mode they sag down to cutoff voltage almost immediately.
As far as I'm aware, the highest genuine capacities come from the Panasonic NCR18650B, which does 3200mah, and are used in Tesla electric vehicles.
There are several different chemistries used on the Cathode, which the lithium ions migrate into as the battery discharges -
Nickel Cobalt Aluminum (NCA)
eg. Panasonic NCR18650B
BEST energy density
BEST lifespan
WORST safety
Nickel Cobalt Manganese (NCM)
eg. Newer power tools
GOOD energy density
AVERAGE lifespan
POOR safety
Cobalt Oxide
eg. older laptops
GOOD energy density
AVERAGE lifespan
WORST safety
Manganese Oxide
eg. older power tools
AVERAGE energy density
POOR lifespan
GOOD safety
Iron Phosphate
POOR energy density
GOOD lifespan
BEST safety
The other half of the battery is the Anode, which stores the lithium ions in their charged stage. Traditionally, this has always been Carbon. However, some batteries with Silicon are starting to appear. These allow the same size cell to store more energy , at the expense of increased weight (little or no gain in power/weight). Silicon has the drawback that it develops micro fractures on charge/discharge , so unlike carbon-anode cells they degrade on use, as well as on storage.
Another development is higher charging voltages. Currently, lithium ion batteries are charged to 4.2V by default, and this is considered "full". In fact however, there are still quite a few lithium ions left on the discharged side of the battery at that point. I don't think they all move over till 4.7V or so. Problem is, the higher up you go, the quicker the electrolyte breaks down (faster calendar degradation). And, the more tetchy, dangerous and unstable the cathode material becomes. They're working on more durable and less flammable electrolytes so that one day we can charge higher...0 -
Yep those panasonic's are pretty good.. Genuine 3.2Ah under a decent load, 3.4Ah at 0.2A (officially).
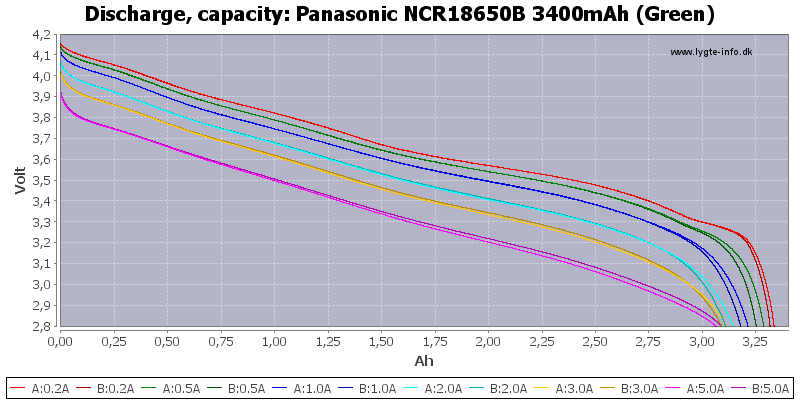
Found quite a few of the older 2.9Ah version in lenovo cells. (snot green colour)0 -
So I should be keeping my iPad below 75% charge as much as possible?
Wife will think I've completely lost the plot if I start keeping it in the fridge!0 -
So I should be keeping my iPad below 75% charge as much as possible?
Wife will think I've completely lost the plot if I start keeping it in the fridge!
I've got a 2 year old Samsung Galaxy Tab 2, which gets used for watching films when working out on a cardio machine at the gym, and i also use it to watch youtube relaxation channel vids in bed. I'd put it on charge before heading to the gym and unplug when it got to 60% - that's enough for a good 5 hours of playback (the newer , thinner models aren't so good). And before bed i'd plug it in , let it get to 30%, start watching camera brushing/whispering/teabag rustling vids till i fall asleep. So naturally i forget to turn it off, then in the morning i dash off to work and my poor tablet would run out of juice and stay empty till the next time i have a bout of insomnia/feel like going to the gym.
Anyway, was last flat was incredibly draughty but it's never been in the fridge, most the time it lives under my bed with the dirty socks. Battery not lost any capacity though.
As for my bike lights, I've had them twelve months but am starting to think they're not long for this world and am trying to work out what I can use the batteries for afterward. I've got a cordless mouse that chews duracells, or maybe i'd like to see what a Remington Nasal Hair trimmer is like on triple the voltage.
Both front and rear lights have recently gotten stuck in what I call "Zombie" mode. I was riding along, and suddenly noticed the main LED was off. Yet the indicator LED on the power button was ON, and colored green, indicating over 50% battery life. However, no combination of short or long presses of the power button would get them to either properly turn on or properly turn off. I eventually managed to fix this by jabbing a thin plastic spatula between the battery end cap and the spring terminal, to cut off power to the control board and reset it. Seems whatever silicon chip that lives in the thing (I'm sure it's got more computer power than we sent men to the moon with..) is starting suffer with vibration and moisture.0

